ARTISAN® Aphakia Clinical Investigation
Intraocular lenses have historically been used as replacements for the natural lens following cataract surgery to correct the condition of aphakia, or the lack of the natural crystalline lens. While aphakic spectacles and contact lenses exist, an ocular implant confers certain benefits to patients. The obvious benefits of intraocular lenses to these patients have been ease of use, good visual acuity, low rates of complications, truer image magnification, and retention of peripheral vision. These attributes have contributed to their increased use throughout the world.
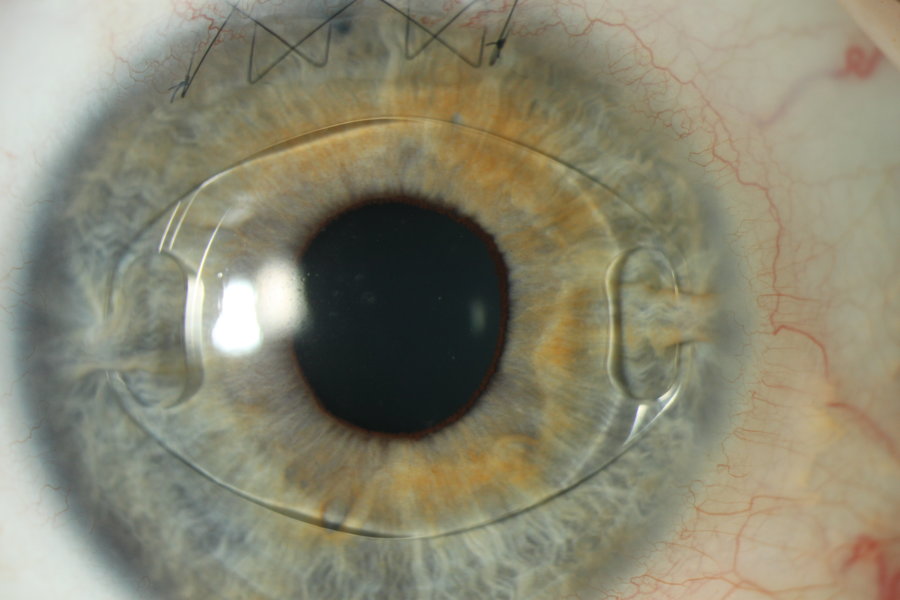
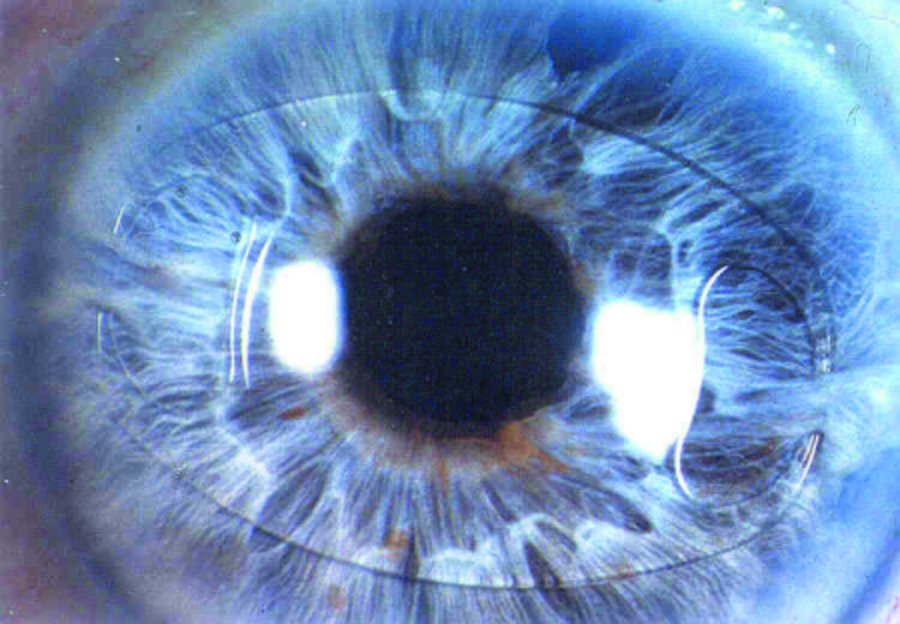
The primary difference between traditional IOLs and the ARTISAN® Aphakia lens is the location and mechanism of the lens placement within the eye. Conventional IOLs are placed behind the iris in the capsular bag. If the capsular bag is not stable, these lenses may alternatively be placed in the ciliary sulcus or sutured to the sclera or iris. Other alternative IOL designs may be placed in the anterior chamber due to lack of capsular support. These anterior chamber IOLs have projections into the anterior chamber angle to support the lens.
The unique design of the ARTISAN® Aphakia lens allows it to be attached to the anterior surface of the iris while maintaining a safe distance from the delicate structures of the anterior chamber. It provides an alternative for posterior IOLs in cases of compromised capsular bags and where angle fixated lenses are deemed an unsuitable option.
The ARTISAN® Aphakia (Iris Claw) lens has been used since its early introduction in 1978 in Europe and Asia for the correction of aphakia following cataract surgery as a primary implant. Reports from surgeons who use the ARTISAN® lens design for the correction of aphakia suggest that these lenses provide good distant visual acuity results and levels of complications comparable to conventional lenses used for the correction of aphakia. The ARTISAN® Aphakia IOL is increasingly being used as the back-up lens of choice by many modern cataract surgeons outside the United States. Approximately 450,000 of these lenses have been implanted worldwide.
It is with this high level of confidence gained by surgeons using this lens in Europe and Asia that further clinical investigations are warranted to bring the availability of this unique lens to the United States. Ophtec USA is currently sponsoring two clinical investigations for ARTISAN® Aphakia lens.
G110050 ARTISAN® Aphakia Lens for the Correction of Aphakia in Adults
G110051 ARTISAN® Aphakia Lens for the Correction of Aphakia in Children
The objective of these clinical investigations is to determine the effectiveness of the ARTISAN® Aphakia lens in the treatment of aphakia and to precisely define the associated risks and, if possible, identify particular groups of patients who may be at high risk of developing complications resulting from the surgical procedure of implanting an ARTISAN® Aphakia lens.
If you are a doctor interested in participating in the trial, Contact us.
If you are a potential patient who would like more information, we can put you in contact with an investigator in your area. Contact us.
Ophtec’s clinical team suggests the following articles for more information on the ARTISAN Aphakia IOL:
ARTISAN® Aphakia IOL Under Investigation As Viable Treatment